Rate Constant Of First Order Reaction Is 6.93 . Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: T 1 /2 = 0.693/k. The rate constant of a first order reaction is 6.93×10−3 min−1.
from chem.libretexts.org
If we start with 10 mol/l, it is reduced to 1.25 mol/l in: The rate constant of a first order reaction is 6.93×10−3 min−1. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. T 1 /2 = 0.693/k. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is.
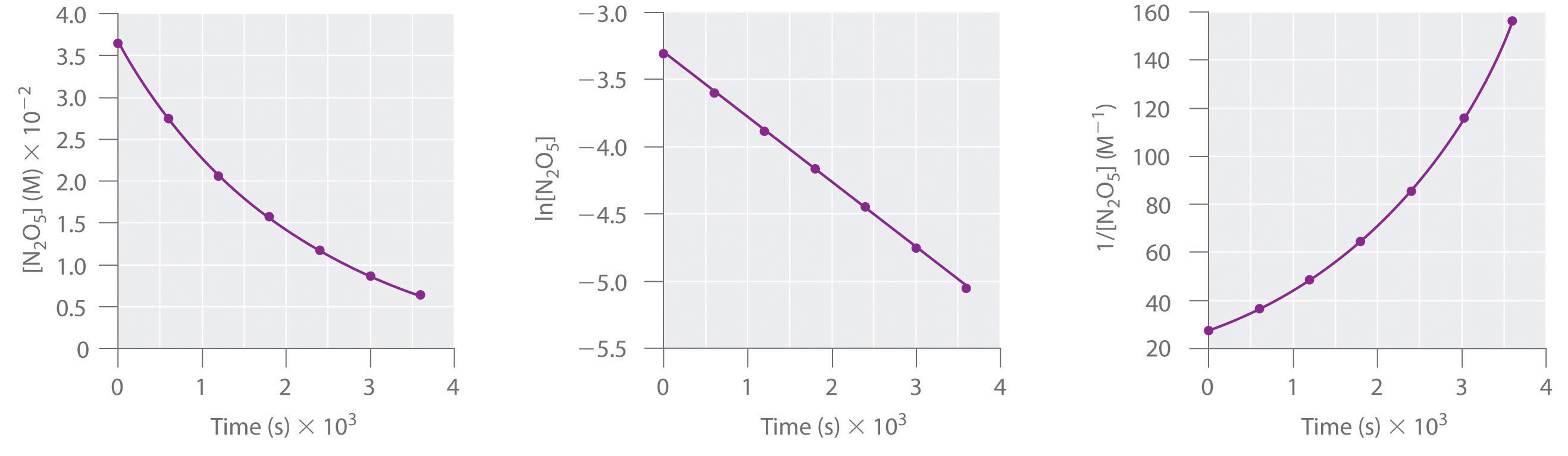
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and
Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. T 1 /2 = 0.693/k. The rate constant of a first order reaction is 6.93×10−3 min−1.
From socratic.org
What is the halflife of a firstorder reaction with a rate constant of Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. The rate constant of a first order reaction is 6.93×10−3 min−1. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web my lecturer mentioned that the formula for the. Rate Constant Of First Order Reaction Is 6.93.
From www.toppr.com
The half life of a first order reaction is 480s . Then, the rate Rate Constant Of First Order Reaction Is 6.93 Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. T 1 /2 = 0.693/k. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. The rate. Rate Constant Of First Order Reaction Is 6.93.
From www.chegg.com
Solved The activation energy of a first order reaction is Rate Constant Of First Order Reaction Is 6.93 The rate constant of a first order reaction is 6.93×10−3 min−1. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: T 1 /2 = 0.693/k. Web differential rate laws are generally used to describe what is occurring on. Rate Constant Of First Order Reaction Is 6.93.
From askfilo.com
CHEMICAL 1. The specific rate constant of first order reaction i.. Rate Constant Of First Order Reaction Is 6.93 Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. The rate constant of a first order. Rate Constant Of First Order Reaction Is 6.93.
From www.youtube.com
The rate constant of a first order reaction is `3 xx 10^(6)" per sec Rate Constant Of First Order Reaction Is 6.93 The rate constant of a first order reaction is 6.93×10−3 min−1. T 1 /2 = 0.693/k. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. If we start with. Rate Constant Of First Order Reaction Is 6.93.
From mavink.com
First Order Reaction Units Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: The rate constant of a first order reaction is 6.93×10−3 min−1. Web my lecturer mentioned that the formula for the. Rate Constant Of First Order Reaction Is 6.93.
From www.youtube.com
Quickvideo Calculating rate constant from a firstorder reaction Rate Constant Of First Order Reaction Is 6.93 Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. The rate constant of a first order reaction is 6.93×10−3 min−1. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. T 1 /2 = 0.693/k. If we start with. Rate Constant Of First Order Reaction Is 6.93.
From saylordotorg.github.io
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant Of First Order Reaction Is 6.93 Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. T 1 /2 = 0.693/k. The rate. Rate Constant Of First Order Reaction Is 6.93.
From chemguru.sg
Rate Equation and Order of Reaction Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. The rate constant of a first order reaction is 6.93×10−3 min−1. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web differential rate laws are generally used to describe what is occurring on. Rate Constant Of First Order Reaction Is 6.93.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. The rate constant of a first order reaction is 6.93×10−3 min−1. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with. Rate Constant Of First Order Reaction Is 6.93.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Rate Constant Of First Order Reaction Is 6.93 Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: The rate constant of a first order reaction is 6.93×10−3 min−1. T 1 /2 = 0.693/k. Web differential rate laws are generally used to describe what is occurring on. Rate Constant Of First Order Reaction Is 6.93.
From askfilo.com
Units of rate constant for a first order reaction. Filo Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: The rate constant of a first order reaction is 6.93×10−3 min−1. Web my lecturer mentioned that the formula for the. Rate Constant Of First Order Reaction Is 6.93.
From brainly.in
expression for the rate constant of the first order reaction Brainly.in Rate Constant Of First Order Reaction Is 6.93 The rate constant of a first order reaction is 6.93×10−3 min−1. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. T 1 /2 = 0.693/k. If we start with. Rate Constant Of First Order Reaction Is 6.93.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant Of First Order Reaction Is 6.93 Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: The rate constant of a first order reaction is 6.93×10−3 min−1. Web differential rate laws are generally used to describe what is occurring on a molecular level during a. Rate Constant Of First Order Reaction Is 6.93.
From www.tessshebaylo.com
Derive An Integrated Rate Equation For Constant The First Order Rate Constant Of First Order Reaction Is 6.93 Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. The rate constant of a first order reaction is 6.93×10−3 min−1. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web differential rate laws are generally used to describe what is occurring on a molecular level during a. Rate Constant Of First Order Reaction Is 6.93.
From byjus.com
What is the unit of rate constant for first order reaction Rate Constant Of First Order Reaction Is 6.93 T 1 /2 = 0.693/k. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. The rate constant of a first order reaction is 6.93×10−3 min−1. Web my lecturer mentioned that the formula for the. Rate Constant Of First Order Reaction Is 6.93.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Rate Constant Of First Order Reaction Is 6.93 Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. If we start with 10 mol/l, it is reduced to 1.25 mol/l in: The rate constant of a first order reaction is 6.93×10−3 min−1. T 1 /2 = 0.693/k. Web differential rate laws are generally used to describe what is occurring on. Rate Constant Of First Order Reaction Is 6.93.
From lavelle.chem.ucla.edu
halflife CHEMISTRY COMMUNITY Rate Constant Of First Order Reaction Is 6.93 If we start with 10 mol/l, it is reduced to 1.25 mol/l in: Web my lecturer mentioned that the formula for the rate constant k for the first order reaction is. Web differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate. The rate constant of a first order. Rate Constant Of First Order Reaction Is 6.93.